Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kitamura, Akira
JAEA-Data/Code 2020-020, 164 Pages, 2021/03
Part of JAEA's Thermodynamic Database (JAEA-TDB) for solubility and speciation of radionuclides (JAEA-TDB-RN) for performance assessment of geological disposal of high-level radioactive and TRU wastes has been updated with subsuming the database for geochemical calculations (JAEA-TDB-GC). This report has focused to update JAEA-TDB-RN after selecting change in standard Gibbs free energy of formation (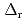
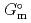 ), change in standard enthalpy change of formation (
), change in standard enthalpy change of formation (
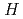

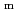 ), standard molar entropy (
), standard molar entropy (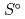
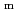 ) and, heat capacity (
) and, heat capacity (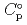 ), change in standard Gibbs free energy of reaction (
), change in standard Gibbs free energy of reaction (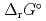
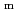 ), change in standard enthalpy change of reaction (
), change in standard enthalpy change of reaction (
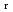
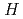

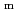 ) and standard entropy change of reaction (
) and standard entropy change of reaction (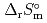 ) as well as logarithm of equilibrium constant (log
) as well as logarithm of equilibrium constant (log
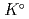 ) at standard state. The extent of selection of these thermodynamic data enables to evaluate solubility and speciation of radionuclides at temperatures other than 298.15 K. Furthermore, the latest thermodynamic data for iron which have been critically reviewed, selected and compiled by the Nuclear Energy Agency within Organisation for Economic Co-operation and Development (OECD/NEA) have been accepted. Most of previously selected log
) at standard state. The extent of selection of these thermodynamic data enables to evaluate solubility and speciation of radionuclides at temperatures other than 298.15 K. Furthermore, the latest thermodynamic data for iron which have been critically reviewed, selected and compiled by the Nuclear Energy Agency within Organisation for Economic Co-operation and Development (OECD/NEA) have been accepted. Most of previously selected log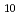
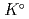 have been refined to confirm internal consistency with JAEA-TDB-GC. Text files of the updated JAEA-TDB have been provided for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
have been refined to confirm internal consistency with JAEA-TDB-GC. Text files of the updated JAEA-TDB have been provided for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
Kitamura, Akira; Yoshida, Yasushi*
Journal of Radioanalytical and Nuclear Chemistry, 327(2), p.839 - 845, 2021/02
Times Cited Count:3 Percentile:44.61(Chemistry, Analytical)Thermodynamic data for radium for radioactive waste management have been predicted using an electrostatic model and correlation with the ionic radii of the alkaline earth metals. Estimation of the standard Gibbs free energy of formation and standard molar entropy of aqueous radium species and compounds has been based on such approaches as extrapolation of the thermodynamic properties of strontium and barium, and use of a model of ion pair formation. The predicted thermodynamic data for radium have been compared with previously reported values.
Kitamura, Akira; Yoshida, Yasushi*; Goto, Takahiro*; Shibutani, Sanae*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 27(2), p.58 - 71, 2020/12
Evaluation and estimation of solubility values are required for a performance assessment of geological disposal of high-level radioactive and TRU wastes. Selection of solubility-limiting solid phases (SSPs) that control the solubility of radionuclides is necessary for the evaluation and estimation of solubility values. The authors have developed a methodology for selection of the SSP through a calculation of saturation indices (SIs) using thermodynamic database to show a transparent procedure for the selection. Literature survey should be performed to confirm decision of the SSP from candidate SSPs which generally have larger SIs from realistic point of view for precipitation and solubility control. The authors have selected the SSPs for the elements of interest for the latest Japanese performance assessment in bentonite and cement porewaters after grouping various water compositions.
Miwa, Shuhei; Nakajima, Kunihisa; Miyahara, Naoya; Nishioka, Shunichiro; Suzuki, Eriko; Horiguchi, Naoki; Liu, J.; Miradji, F.; Imoto, Jumpei; Afiqa, B. M.; et al.
Mechanical Engineering Journal (Internet), 7(3), p.19-00537_1 - 19-00537_11, 2020/06
We constructed the fission product (FP) chemistry database named ECUME for LWR severe accident. This version of ECUME is equipped with dataset of the chemical reactions and their kinetics constants for the reactions of cesium(Cs)-iodine(I)-boron(B)-molybdenum(Mo)-oxygen(O)-hydrogen(H) system in gas phase, the elemental model for the high temperature chemical reaction of Cs with stainless steel applied as the structural material in a reactor, and thermodynamic data for CsBO vapor species and solids of Cs
vapor species and solids of Cs Si
Si O
O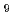 and CsFeSiO
and CsFeSiO for these chemical reactions. The ECUME will provide estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
for these chemical reactions. The ECUME will provide estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
Kitamura, Akira
Nihon Genshiryoku Gakkai-Shi ATOMO , 62(1), p.23 - 28, 2020/01
, 62(1), p.23 - 28, 2020/01
Thermodynamic databases (TDBs) for performance assessment of geological disposal of high-level waste and TRU waste have been developed to predict solubility and speciation of radionuclides in groundwater in some countries including Japan. The present manuscript briefly describes current status of development of the TDB organized by the Nuclear Energy Agency within the Organisation of Economic Co-operation and Development (OECD/NEA) and the TDBs in some countries including Japan.
 -UO
-UO
 -CO
-CO
 system and integration with JAEA's thermodynamic database for geochemical calculations
system and integration with JAEA's thermodynamic database for geochemical calculationsKitamura, Akira
JAEA-Data/Code 2018-018, 103 Pages, 2019/03
The latest available thermodynamic data were critically reviewed and the selected values were included into the JAEA-TDB for performance assessment of geological disposal of high-level radioactive and TRU wastes. This critical review specifically addressed thermodynamic data for (1) a zirconium-hydroxide system through comparison of thermodynamic data selected by the Nuclear Energy Agency within the Organisation for Economic Co-operation and Development (OECD/NEA), (2) complexation of metal ions with isosaccharinic acid based on the latest review papers. Furthermore, the author performed (3) tentative selection of thermodynamic data on ternary complexes among alkaline-earth metal, uranyl and carbonate ions, and (4) integration with the latest version of JAEA's thermodynamic database for geochemical calculations. The internal consistency of the selected data was checked by the author. Text files of the updated and integrated thermodynamic database have been prepared for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
Rai, D.*; Yui, Mikazu; Kitamura, Akira
Progress in Nuclear Science and Technology (Internet), 5, p.19 - 26, 2018/11
The objectives of this presentation are (1) to describe the solubility method, (2) to list desirable criteria of the solubility method so that the reader can recognize which studies have been done in a way that yields quality information, (3) to present an example of how to use the evaluation criteria, and (4) to provide a few examples of future research needs where the solubility method is ideally suited and the other methods are unsuitable for these investigations.
 (am) solubility at 25
(am) solubility at 25 C in the Ca
C in the Ca -Na
-Na -H
-H -Cl
-Cl -OH
-OH -H
-H O system; A Critical review
O system; A Critical reviewRai, D.*; Kitamura, Akira; Altmaier, M.*; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Journal of Solution Chemistry, 47(5), p.855 - 891, 2018/05
Times Cited Count:9 Percentile:9.27(Chemistry, Physical)We have critically reviewed experimental data for Zr hydrolysis constant values for formation of several mononuclear and polynuclear species and a solubility product value for ZrO (am). We have determined new/revised values for the formation constants of Zr(OH)
(am). We have determined new/revised values for the formation constants of Zr(OH)
 , Zr(OH)
, Zr(OH) (aq), Zr(OH)
(aq), Zr(OH)
 , Zr(OH)
, Zr(OH)
 and Ca
and Ca Zr(OH)
Zr(OH)
 , and the solubility product for ZrO
, and the solubility product for ZrO (am) after the critical review.
(am) after the critical review.
 (cr)
(cr)Rai, D.*; Kitamura, Akira; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Radiochimica Acta, 104(8), p.583 - 592, 2016/08
Times Cited Count:5 Percentile:43.12(Chemistry, Inorganic & Nuclear)Solubility studies were conducted with HfO (cr) solid as a function of acid concentrations. These studies involved (1) using two different amounts of the solid phase, (2) acid washing the bulk solid phase, (3) preheating the solid phase to 1400
(cr) solid as a function of acid concentrations. These studies involved (1) using two different amounts of the solid phase, (2) acid washing the bulk solid phase, (3) preheating the solid phase to 1400  C, and (4) heating amorphous HfO
C, and (4) heating amorphous HfO (am) suspensions to 90
(am) suspensions to 90  C to ascertain whether the HfO
C to ascertain whether the HfO (am) converts to HfO
(am) converts to HfO (cr) and to determine the solubility from the oversaturation direction. Based on the results of these treatments it is concluded that the HfO
(cr) and to determine the solubility from the oversaturation direction. Based on the results of these treatments it is concluded that the HfO (cr) contains a small fraction of less crystalline, but not amorphous, material [HfO
(cr) contains a small fraction of less crystalline, but not amorphous, material [HfO (lcr)] and this, rather than the HfO
(lcr)] and this, rather than the HfO (cr), is the solubility-controlling phase in the range of experimental variables investigated in this study. The solubility data are interpreted using both the Pitzer and SIT models. The log
(cr), is the solubility-controlling phase in the range of experimental variables investigated in this study. The solubility data are interpreted using both the Pitzer and SIT models. The log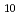 of the solubility product of HfO
of the solubility product of HfO (cr) is estimated. The observation of a small fraction of less crystalline higher solubility material is consistent with the general picture that mineral surfaces are often structurally and/or imperfect leading to a higher solubility than the bulk crystalline solid. This study stresses the urgent need, during interpretation of solubility data, of taking precautions to make certain that the observed solubility behavior for sparingly-soluble solids is assigned to the proper solid phase.
(cr) is estimated. The observation of a small fraction of less crystalline higher solubility material is consistent with the general picture that mineral surfaces are often structurally and/or imperfect leading to a higher solubility than the bulk crystalline solid. This study stresses the urgent need, during interpretation of solubility data, of taking precautions to make certain that the observed solubility behavior for sparingly-soluble solids is assigned to the proper solid phase.
Department of Fuel Cycle Safety Research
JAERI-Review 2001-019, 108 Pages, 2001/07
Department of Fuel Cycle Safety Research, JAERI, has been carrying out research on safe and rational disposal systems of radioactive wastes arising from medical activities and research institutes (RI and Research Institute Waste). The research area includes a study on molten solidified waste form, a geological survey on Japan, a proposal on integrated disposal systems, data acquisition for safety evaluation, and a safety analysis of disposal systems. This report introduces progress and future works for the treatment and disposal of RI and Research Institute Waste.
Kobayashi, Taishi*; Sasaki, Takayuki*; Kitamura, Akira
no journal, ,
The solubility of U(OH) (am) was investigated in the presence of isosaccharinic acid (ISA) as a function of hydrogen ion concentration (pHc) and ISA concentration. The solubility in neutral pH region was independent of pHc, while those in alkaline pH region slightly increased with increasing pHc. The solubility was investigated as a function of ISA concentration and found to increase with a slope of approximately 2 with increasing ISA concentration. It was therefore suggested that U(OH)
(am) was investigated in the presence of isosaccharinic acid (ISA) as a function of hydrogen ion concentration (pHc) and ISA concentration. The solubility in neutral pH region was independent of pHc, while those in alkaline pH region slightly increased with increasing pHc. The solubility was investigated as a function of ISA concentration and found to increase with a slope of approximately 2 with increasing ISA concentration. It was therefore suggested that U(OH) (ISA)
(ISA)
 and U(OH)
and U(OH) (ISA)
(ISA)
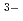 were the dominant U(IV)-ISA complexes, which were similar to those observed in Zr(IV)-ISA system. Complex formation constants were determined by the least square fitting analysis of the solubility data and the calculated solubility curve well reproduced the experimental data.
were the dominant U(IV)-ISA complexes, which were similar to those observed in Zr(IV)-ISA system. Complex formation constants were determined by the least square fitting analysis of the solubility data and the calculated solubility curve well reproduced the experimental data.